| Phone |
| |
| |
| 대표전화 |
1577-7956 |
| 본 사 |
031-457-9187 |
| 대 전 |
042-932-1265 |
| |
|
| Web Site Visitors |
| |
| |
| Today |
569 |
| Yesterday |
1221 |
| Since 2006 |
2,437,001 |
| |
|
 |
|
| 영진코퍼레이션 종합카다로그 |
|
|
 |
|
| 리스트돌아가기 |
Application Note 원문보기 | |
|
| (참조논문) A Nanostructured Lipid Carrier for Delivery of a Replicating Viral RNA Provides Single, Low-Dose Protection against Zika.
Jesse H. Erasmus, et al. Molecular Therapy Volume 26, ISSUE 10, P2507-2522, October 03, 2018 |
INTRODUCTION
차세대 RNA 백신은 2000년대 초부터 백신연구의 주관심사였다.
이는 antigen 의 생산이 복잡한 생산과정을 거치지 않기 때문에 팬데믹과 같은 상황에서는 특히 유용한 tool 이다.
그러나 RNS 자체는 백신으로서 매우 불안정하기 때문에 특별한 delivery system 이 필요한데 이를 위해
다양한 바이러스성 백터와 비바이러스성 nano particle 을 carrier 로 활용하기 위한 연구가 많이 이루어졌다.
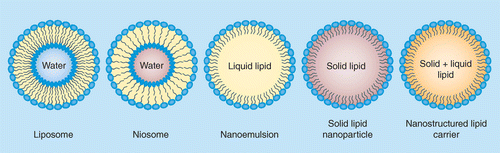
Lipid nanoparticles (LNP), cationic nanoemulsions (CNE) 그리고 nanostructured lipid carriers (NLC) 등이
가장 활발히 연구가 이루어졌으며 가장 가능성이 있어보인다.
RNA 분자를 delivering 하기 위해 nanoparticle 을 이용할 경우에는 in-situ encapsulation 과 post adsorption
두가지 방법이 많이 사용된다. 후자의 경우에는 two-step 으로 주로 이루어지며 다음 논문에서 자세히 소개되어있다.
A Nanostructured Lipid Carrier for Delivery of a Replicating Viral RNA Provides Single, Low-Dose Protection against Zika.
이 application note 에서는 이 논문을 요약하고 왜 이 방법이 백신 대량생산과 현재의 팬데믹 상황에
대응하기에 가장 적합한 지를 소개하고자 한다.
RNA VACCINES
전통적인 바이러스 백신은 계란에서 배양하는 방식이고 2세대 백신은 subunit protein 백신이고 주로 박테리아세포에서 배양된다.
이러한 생산 방법들은 생산 및 추출 공정을 optimization 하기가 어렵고 또 validation 하는데 기간이 많이 소요된다.
반면 핵산 기반의 백신들은 훨씬 빠른 시간에 개발 생산할 수 있다는 장점이 있으나 이 백신물질들은 인체내에서
매우 불안정하므로 이 백신물질을 인체내로 안전하게 전달하는 delivery system 개발이 매우 중요하다.
DELIVERY OF RNA WITH NANOSTRUCTURED LIPID CARRIERS (NLCs)
LNPs, CNEs, 그리고 NLCs 와 같은 다양한 lipid-based nanoformulations 들은 direct encapsulation 또는 post adsorption
방법 중 하나를 이용하여 RNA delivery 에 사용되고 있다.
encapsulation 방법은 LNP 가 백신물질과 함께 동일한 vial 에서 제조되는데 이로 인해 formulation 의 stability 와 scalability 에 문제가 있다.
adsorption 방법은 delivery system 을 대량으로 생산할 수 있고 팬데믹을 대비하여 저장할 수 도 있다.
pathogen 이 특정되어지면 RNA antigen 을 개발하여 대량으로 만들 수 있다.
그리고 나서 RNA 를 NLC formulation 에 넣게되면 RNA 는 particle 표면에 binding 하게 된다.
이러한 two-step 방법은 LNP 방법에 비해 더 다양한 대안이 될 수 있으며 팬데믹 상황에 신속히 대응할 수 있는
가장 이상적인 방법이다.
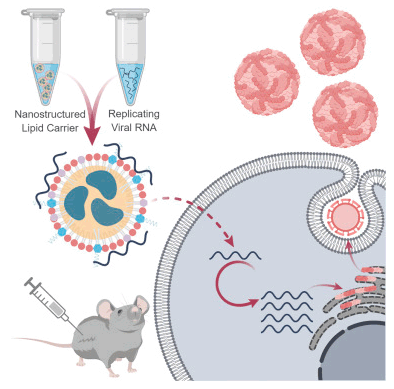
저자는 Zika 백신의 delivery system 으로서 Nonstructured Lipid Carrier (NLC) 를 플랫폼으로 개발하였다.
NLC FORMULATION
이 논문에서 개발된 NLC 는 다음 성분으로 구성된 hybrid formulation 이다.
- 스쿠알렌 오일 : NLC 의 core 로 작용하며 squalene-based emulsion 제조에 수십년간 사용되어 온
Microfluidizer 장비를 이용하여 제조하였다.
- Dynasan 114 : solid phase lipid 로서 인체내에서 circulation time 을 증대시키는 기능을 한다.
- Span 60 & Tween 80 : nanoparticle 을 안정화시키고 그것의 bioavailability 를 향상시키는 역할을 한다.
- DOTAP (cationic phospolipid Dioleoyl-3-trimethylammonium propane) : 양전하를 띈 RNA 가 잘 binding 할 수 있게 만드는 기능.
이 hybrid core formulation 은 nanoparticle 의 stability 가 매우 탁월하여 제조가 매우 용이하다.
NLC FABRICATION
nanoparticle 제조에서 가장 이상적인 방법은 다음과 같은 특성을 가지고 있어야 한다.
- 원하는 입자크기를 쉽게 만들수 있어야 한다.
- stability 가 탁월해야 하다.
- filter 를 하였을 때 멸균처리가 가능해야 한다.
- 다양한 생리화학적 특성을 가진 NLC 제조가 가능해야 한다.
- 대량생산이 가능해야 하다.
Microfluidizer® Technology 는 이러한 모든 요구조건을 모두 충족시킬 수 있다.
이번 연구에서 모든 NLC formulation 은 M110P Microfluidizer® 를 이용하여 제작되었으며
30,000psi 에서 5 pass 조건을 이용하였다.

Figure 1 - M110P Microfluidizer processor
RESULTS
모든 sample 에서 평균입자크기가 100nm 이하인 매우 균일한 NLC 를 구하였으며 filter 를 이용하여 멸균하였다.
self-replicating RNA-encoding Zika virus antigent 을 만든 후 이 RNA 를 final NLC 에 adding 하였다.
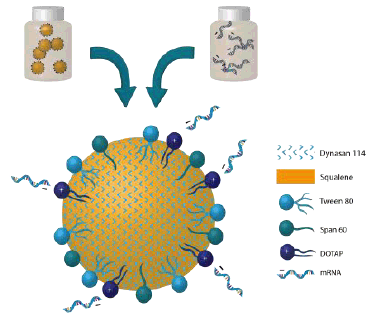
Figure 2 - Schematic representation of RNA surface adsorbed to NLC nanoparticle post-admixture
NLC 제조 공정이 확립된 후 조성 성분들의 loading capacity 와 safety & immunogenecity 들을 optimization 하였는데
10mg/ml 농도에서 RNA binding 이 100% 이루어졌다.
이 article 은 10ng 의 아주 극소량의 RNA 를 함유한 dose single 에 의해서도 Zika virus 감염을 방지할 수 있는
충분한 면역력을 유도할 수 있다는 것을 보여주었다.
|
|
|
|







