| Phone |
| |
| |
| 대표전화 |
1577-7956 |
| 본 사 |
031-457-9187 |
| 대 전 |
042-932-1265 |
| |
|
| Web Site Visitors |
| |
| |
| Today |
628 |
| Yesterday |
1370 |
| Since 2006 |
2,410,880 |
| |
|
 |
|
| 영진코퍼레이션 종합카다로그 |
|
|
INTRODUCTION
liposome 은 약물질과 백신물질을 전달하는데 널리 사용되고 있다.
전통적인 liposome 제조는 sovent 를 이용하는 것인데 이 경우 solvent 를 제거하는 몇가지 공정이 추가되는데 인체에 대한 유해성과 안전성에 문제가 될 수 있다.
최근 solvent 를 사용하지 않는 새로운 방법이 개발되었는데 이 내용을 소개하고자 한다. (페이지 상단 참조논문 확인)
이 paper 에서는 고효율 drug encapsulation level 을 유지하면서도 solvent-free mass production 방법을 설명하고 있다.
|
 |
|
THE PROBLEM
전통적인 liposome 제조 방식은 유기용매를 사용하는 것이다.
그러나 유기용매의 사용은 안전성과 인체 유해성 그리고 환경 등의 문제가 많아 바람직한 것은 아니다.
제약분야에서 허용되는 유기용매의 사용량은 ICH Q3 guideline 에 정의되고 있다.
liposome 제조에 널리 사용되는 클로로포름과 메탄올 같은 유기용매는 class 2 solvent 로서 ICH Q3 guideline 에서는 사용 금지로 분류되고 있다.
그리고 저독성 유기용매로 분류되는 에탄올 같은 경우도 5000ppm 또는 0.5% 이상을 초과해서는 안된다.
THE SOLUTION
최근 Strathclyde 대학교의 연구진들이 유기용매를 사용하지 않는 liposome 제조방법을 개발하였다.
이 방법은 microfluidizer technology 를 이용하는 것이다.
먼저 powdered lipid 를 aqueous buffer 에 넣고 mixing 한 후 Microfluidizer M110P 를 이용하여 처리하는 것으로
개략적인 공정도는 다음과 같다.
Microfluidizer 장비를 이용하면 크기가 매우 미세하면서도 크기가 일정한 liposome vesicle 을 만들 수 있으면 또한 대량 생산도 편리하게 할 수 있다는 장점이 있다.
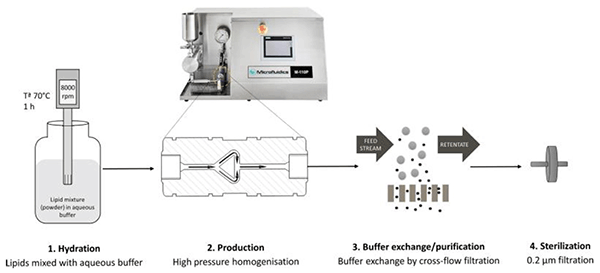
Figure 1 - Schematic representation of liposome manufacture, buffer exchange/purification, drug loading and sterilization
THE RESULTS
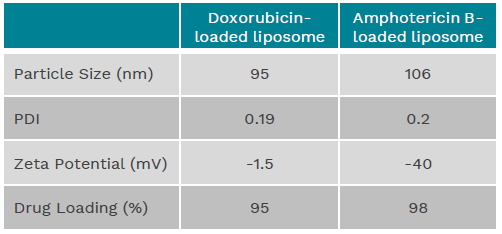
Table 1 - Physicochemical characterizations of doxorubicin-loaded and amphotericin B-loaded liposomes prepared via the solvent-free method
이러한 sovent-free 공정을 이용하여 doxorubicin-loaded PEGylated liposome 과 amphotericin B-loaded liposome 제조한 결과
두개 모두 liposome 크기가 100-110nm 이었으며 입도균일도를 표시하는 polydispersity index (Pdi) 는 < 0.2 이하로서 liposome 크기가
매우 균일하게 제조 되었다는 것을 확인하였다.
또한 encapsulation efficiency 역시 90% 이상으로 확인되었다.
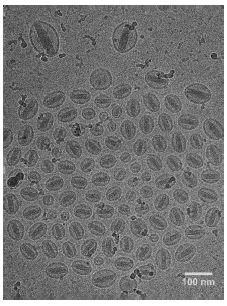
Figure 2 - CryoTEM image of doxorubicin-loaded liposome vesicles produced by a Microfluidizer® processor using the solvent-free approach
Fig 2 는 약물질 doxorubicin 이 excapsulation 되어진 unilamellar liposome vessicle 이 매우 균일한 크기로 존재하고 있다는 것을 보여주는 사진이다.
연구팀들은 tangetial flow filtration 과 sterile filtration 과정을 포함하는 전체 제조 공정에서 liposome 의 입자크기, PDI 그리고 약물질의 loading 상태가
변하지 않고 안정하게 유지되었다고 이야기 한다.
또한 doxorubicin-loaded liposome 의 drug-release testing 결과 6시간 경과 후 60% 의 doxorubicin release 를 확인하였다.
|
|
|
|







