| Phone |
| |
| |
| 대표전화 |
1577-7956 |
| 본 사 |
031-457-9187 |
| 대 전 |
042-932-1265 |
| |
|
| Web Site Visitors |
| |
| |
| Today |
378 |
| Yesterday |
1114 |
| Since 2006 |
2,414,613 |
| |
|
 |
|
| 영진코퍼레이션 종합카다로그 |
|
|
 |
Publication |
| |
| |
D. Lerche, T. Sobisch, L.U.M Gmbh, Colloids and Surfaces A : Physicochem. Eng Aspects 440 (2014) 122-130 |
| |
 |
Introduction |
| |
| |
분산한 물질의 분산상태 (dispersion state) 는 공정중의 중간 생성물의 특성, 최종 생산품의 기능적인 많은 특성 그리고 제품의 shelf life 에 큰 영향을 미친다.
이것은 분산한 물질이 flocculation, agglutination or agglomeration 그리고 coalescence 등의 현상이 일어나 분산한 물질이 원래의 모습으로 되돌아 가는 현상
즉 destabilization 일어나게 되어 제품의 특성이 바뀌게 되기 때문이다. Flocculation or agglomeration 은 입자들 사이의 weak physical interaction 에 의해
서로 모이게 되는 가역적 현상으로서 약한 기계적인 힘을 가하여 주면 재분산이 가능하다.
전통적으로 분산한 물질의 안정성은 입자의 zeta potential 을 측정하여 입자간 electrostatic interaction 을 계산하여 분석하는 방법을 사용하여왔다.
즉 절대적 zeta potential 값이 10mV 이하이면 flocculation 이 일어나고 30mV 이하이면 분산상태가 불안정하다는 것을 의미하고 ±30mV 이상에서는
분산이 안정한 상태로 있다는 것을 의미한다. 그러나 zeta potential 에 의한 분산안정성 분석은 hard sphere 의 particle 에서만 적용 가능하다는 것을
고려하여야 한다. 이러한 hard sphere particle 은 (1) potential 이 입자 표면에 주로 위치해 있고 (2) surface conductivity 가 없고 (3) 입자크기에 비해 얇은
diffuse double layer 를 가지며 (4) 3D-surface structure 가 없는 특징이 있다.
나노분산과 같은 formulation 에서는 표면의 기능성뿐만 아니라 steric and entropic stabilization 이 분산에 더 큰 영향을 미친다. 이 경우 입자는 주로
polyelectrolytes 또는 amphoteric surface molecules 로 이루어진 ion-permeable surface layer 에 의해 둘러 쌓여 있으며 이 molecule 의 head group 이
외부로 향하고 있어 바이오의 세포와 유사한 형태를 뛰고있다. 이런 형태의 입자를 soft particle 이라고 부르는데 hard particle 과의 가장 큰 차이점은
(1) core particle 주위에 fixed electric charges 가 3차원으로 분포되어 있는 층이 추가로 존재한다는 것이며 (2) 이로 인해 hydrodynamic flow 와
electric conductivity 특성을 가진다는 것이다. 이러한 ion-penetrable layer 를 particle corona 라 부른다. 이러한 soft particle 의 zeta potential 은 실험적
electrophoretic mobility data 에 의하는 기존의 zeta potential 측정방식으로는 구할 수 없으며 soft particle 사이에 작용하는 interaction potential 도 예측할 수 없고
또한 분산안정성 여부도 예측할 수 없는 것이다.
이 논문에서는 particle-particle interaction 을 확인하는 새로운 접근 방법인 analytical
centrifugation (STEP Technology) 을 소개하고 분산한 물질의 phase separation 을 확인하는데 있어 zeta potential 과 어떤 차이점이 있는지 확인하고자 한다.
Hard electronically stabilized particle 의 경우에는 zeta potential 측정법과 analytical centrifugation 방법 모두 동일한 결과를 보였으나 soft particle 의 경우에는
analytical centrifugation 방법이 phase separation 을 in situ visualization 할 수있으며 또한 flocculation 상태를 정확히 구별할 수 있었다.
|
| |
 |
Analytical centrifugation |
| |
| |
분산한 물질을 적절한 tube 에 넣고 회전시켜 원심력을 가하면서 tube 와 평행하여 Near Infra-red (근적외선) 를 조사하고 tube 반대편에서 약 2000개의
sensor 로서 투과되어지는 NIR 의 양 (Transmission) 을 측정하여 그래프로 표시하면 tube 전체 위치에서 물질의 농도 변화를 나타내는 Transmission graph 를
그릴 수 있다. 정해진 시간별로 연속적으로 측정되어지는 모든 그래프를 하나의 도표에 나타내면 시간의 변화에 따른 transmission 의 변화를 한눈에 볼 수 있는
transmission profile 을 구할 수 있다. 이런 방법을 STEP Technology (Space & Time resolved Extinction Profile) 이라 하는데 이 기법을 이용하면 원심력하에서
분산한 물질의 미세한 phase separation 변화를 확인할 수 있다. |
| |
 |
Example for the classical case of electrostatically stabilized metal oxide dispersion |
| |
| |
|
40mV (at pH=5), close to zero (at pH=9) 그리고 below -20mV (at pH=11) 의 zeta potential 을 가지고 입자크기는 100nm 에서 200nm 사이에 분포하는
AEROXIDE Alu C 를 각각 pH=5, 9, 11 농도를 가진 용액에서 분산시켜 analytical centrifugation 하여 분산상태를 확인하였다 (Fig.1).
AEROXIDE Alu C 는 대표적인 hard surface particle 이라 할 수 있다.
Fig.1 의 a profile 은 pH=5 and ZP=40mV 하에서 입자들은 각각의 입자크기에 따라 각기 다른 속도로 일정하게 sedimentation 이 일어나는 것을 알 수 있는데
원심력하에서 이러한 형태의 phase separation 이 일어나는 것은 dispersion 이 매우 stable 한 상태에 있다는 것을 의미하고 또한 다양한 입자크기로
이루어진 polydispersion 인 것을 의미한다.
Isoelectric point 인 pH=9 조건에서 분산한 물질들은 개별 입자가 아닌 flocculation 되어진 입자 형태로 존재하게 된다 (Fig.1 b.).
Transmission profile 이 매우 가파르고 우측으로 가면서 profile 사이 간격이 점점 더 좁아지는 것을 알 수 있는데 이는 flocculation 상태로 존재하는 입자들이
침강하면서 원심력에 의해 compression 되어지기 때문이다. 결국 pH=9 하에서는 분산 물질이 매우 불안정하게 존재한다는 것을 알 수 있다.
pH=11 에서 Alu C 입자의 zeta potential 이 -20mV 이하가 되는 분산 물질의 analytical centrifugation 결과를 보면 (Fig.1 c) Fig.1 a transmission profile 과는
다른 모습을 모이고 있다.
Fig.1 a 에 비해 각 profile 간 간격이 조금 더 넓고 우측에서 profile 이 compression 되는 것을 볼 수 있는데 이는 분산한 물질에 개별 입자와 flocculation 된
입자가 동시에 존재한다는 것을 의미한다.
이 결과로부터 우리는 zeta potential 보다 analytical centrifugation 이 분산물질의 flocculation 형성 여부, sediment packing density 등과 같은 실제적인
정보를 더 많이 제공해주고 있다는 것을 알 수 있다.
|
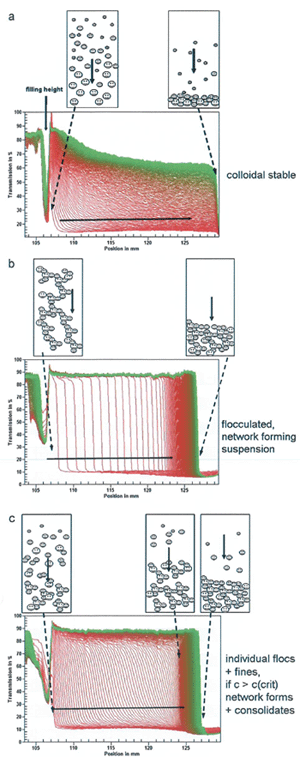
Fig.1. Transmission profiles of AEROXIDE Alu C |
|
| |
 |
Example for electrostatically stabilized metal oxide dispersions < 100nm |
| |
| |
Particle size 가 100nm 이하가 되면 zeta potential value 가 크면 분산 물질의 stability 도 증가한다는 상관관계의 연관성이 급격하게 줄어들게 된다.
30nm 에서 100nm 사이 크기의 입자로 이루어진 Titania (AEROXIDE P25) 의 zeta potential 은 Fig.2 와 같으며 pH 2, 4, 5, 7, 9 그리고 10 에서의
Titania 분산물의 analytical centrifugation 결과는 Fig. 3 과 같다. pH 2 에서는 50mV 의 zeta potential 에서 예측할 수 있듯이 이 분산물질은 매우
stable 하며 개별 입자 크기에 따라 각기 다른 velocity 로 sedimentation 이 (polydisperse) 일어나고 있다는 것을 알 수 있다.
약 48mV 의 Zeta potential 을 가지는 pH 4 에서도 stable 한 분산 상태를 알 수 있으나 pH 2 에 비해서 profile 간 간격이 더 넓게 나타나는데
이는 비록 zeta potential 은 pH 2 와 비슷한 수준이나 입자들이 aggregation 되어 sedimentation 이 더 빨리 이루어지고 있다는 것을 알 수 있다.
pH 5 에서는 zeta potential 이 30mV 로 줄어들게 되지만 여전히 높은 수치이다. 하지만 dispersion state 는 매우 불안하여 입자들의 flocculation 형성이
강하게 일어나고 시간이 지날수록 flocculation 이 심하게 compression 되는 것을 알 수 있다.
Isoelectric point 인 pH 7 에서는 더 크고 더 빨리 sedimentation 되는 flocculation 이 형성 되었으며 flocculation network 가 compression 되는 현상은
일어나지 않는다는 것을 알 수 있다.
Charge 가 바뀌어지는 pH 9 와 10 에서는 flocculation network 가 compression 되는 현상이 다시 나타나고 특히 pH 10 에서는 flocculation 되지 않은
개별 입자들이 sedimentation 되고 있다는 것을 알 수 있다.
|
| |
 |
Enzyme covered silica nanoparticles |
| |
| |
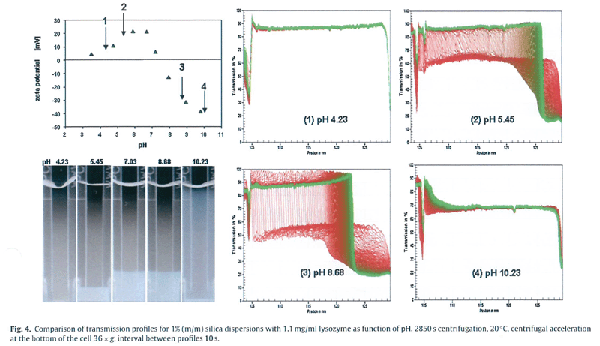
여기서는 전형적인 soft particle 의 예를 소개한다.
IEP 3 이고 입자 크기가 20nm 인 silica particle 을 직경 1.9nm, IEP 10.7 인 lysozyme 으로 coating 하였으며 coated particle 의 pH 에 따른
zeta potential 을 Fig. 4 에 나타내었다.
Plain silica 의 IEP 인 pH 3 이상에서 positively charged lysozyme 과의 상호작용에 의해 pH 6.5 에서 zeta potential 이 >20mV 로 최대가 된다.
pH 4.23, 5.45, 8.68 그리고 10.23 에서의 zeta potential 은 각각 5, 15, -30 그리고 -40mV 가 되며 이때의 analytical centrifugation 결과를 Fig. 4 에 나타내었다.
Zeta potential 원리에 의하면 pH 4 에서는 낮은 stability, pH 7 로 가면서 stability 가 증가하고 pH 8.5 로 가면서는 다시 stability 가 감소하고 pH 9 이상에서는
high stability 를 보여야 한다. 그러나 analytical centrifugation 결과는 완전히 다른 내용을 말하고 있다.
pH 4.23 에서는 transmission profile 의 변화가 거의 없으며 또한 transmission 이 매우 높은 값으로 나타나고 있는데 이는 20nm 의 coated silica 입자가
완전히 개별적으로 존재하고 aggregation 이 소량만 일어나고 있다는 것을 의미한다. 이는 비록 zeta potential 값이 매우 낮음에도 불구하고 dispersion 이
매우 stable 하게 존재한다는 것을 의미한다. pH 가 4.23 에서 5.45 로 되면서 zeta potential 이 증가하였음에도 불구하고 dispersion 내에서 flocculation 이
일어나 sedimentation 이 활발히 일어나고 있으며 결국은 dispersion 이 매우 불안정 하다는 것을 알 수 있다.
또한 pH 가 8.68 에서 zeta potential 이 약 -35mV 가 되었음에도 불구하고 zeta potential 이 약 15mV 인 pH 5.45 에서 보다 dispersion stability 가 훨씬
더 감소했다는 것을 알 수 있다.
pH 10.23 인 조건에서는 입자들 사이의 interaction 이 감소해 flocculation 형성이 줄어들어 다소 안정적인 분산 상태를 유지한다는 것을 알 수 있다.
|
| |
 |
Polyelectrolyte coated magnetic nanoparticles for gene delivery |
| |
| |
Polyelectrolyte 로 coating 된 core-shell type silica/iron oxide magnetic nanoparticles 는 매우 복잡한 구조를 가지고 있다.
Surface phosphate group 과 polyelectrolyte 를 가진 silicon dioxide 가 layer 를 형성하며 magnetic core 를 둘러 싸고 있다.
이러한 구조에 의해 전형적인 two-dimensional surface charge distribution 이 아닌 특이한 spatial fixed charge distribution 구조가 된다.
Dispersion 에서 이러한 polyelectrolyte 들이 flocculation 을 형성하는 과정은 매우 복잡하다.
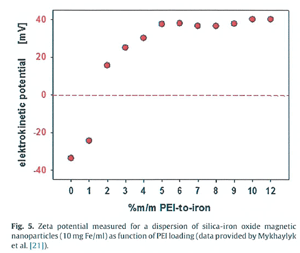
Negatively charged silica-iron oxide magnetic nano particle 을 positively charged polyethylene Imine (PEI) 으로 PEI / iron (%m/m) 으로 정량적으로 coating
하게 되면 particle 의 charge 가 변하고 zeta potential 도 Fig. 5 에 보여주듯이 undecorated particles (SO-Mag5) -38±2 mV 에서부터 SO-Nag6-5
약 40mV 까지 다양하게 변하게 된다.
이 경우 zeta potential 은 dispersion 에 영향을 미치지 않고 iron 당 PEI 농도가 10%, 12% 일 때 가장 적절한 dispersion 을 구할 수 있다.
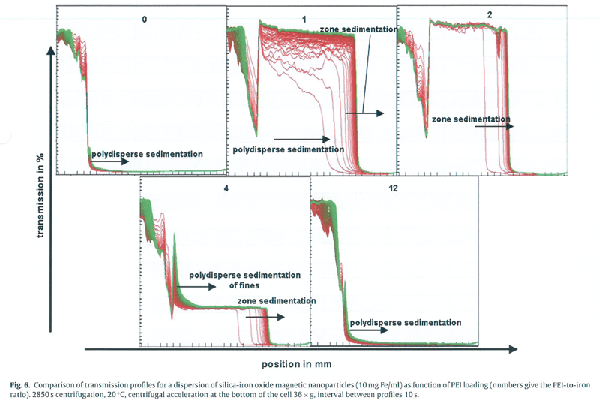
PEI decoration 에 대한 dispersion 의 stability 를 Analytical centrifuge 를 이용하여 분석하였다 (Fig. 6)
PEI 를 첨가하지 않은 undecorated SO-Mag5 의 경우 (Fig. 6, 0번 graph) 변화가 거의 없을 정도로 매우 stable 한 dispersion 임을 알 수 있다.
1% 의 PEI 를 첨가한 경우 (Fig. 6, 1번 graph) net negative charge 가 다소 감소하였으나 dispersion 상태가 많이 변하였다. 즉 개별 입자들이
sedimentation 하다가 입자사이에 flocculation 이 형성되어 입자가 한꺼번에 떨어지는 형상을 보인다. 2% 의 PEI 를 첨가한 경우 (Fig. 6, 2번 graph) 개별 입자의
이동은 보이지 않고 거의 전부가 flocculation 되어 network 형식으로 sedimentation 되어 compression 되는 zone sedimentation 형상이 나타난다.
2% 의 PEI 를 첨가한 경우 (Fig. 6, 2번 graph) sedimentation pattern 이 다시 변하는데 특이하게도 두 가지 종류의 입자가 sedimentation 하는 bimodal separation 과
유사한 형태를 나타내고 있다. 즉 초기에는 다양한 크기의 입자들이 개별적으로 sedimentation 하는 형태를 보이다가 과정이 진행함에 따라 zeta potential 이
30mV 이상임에도 불구하고 개별입자들이 다시 상호작용하여 flocculation network 를 형성하여 모두 같이 떨어지는 zone sedimentation 모습을 보이고 있다.
PEI-loading 양을 더욱 늘려 총 12% 까지 PEI 양을 증가시켜도 (Fig. 6, 12번 graph) zeta potential 은 일정하게 40mV 를 유지하는데 flocculation 은 일어나지 않고
전체적으로 polydisperse 형태를 나타내어 매우 안정한 dispersion 을 유지하고 있다.
|
| |
 |
Conclusion |
| |
| |
자연중력 또는 원심력 하에서 dispersion 의 separation 되는 것을 관찰하면 particle surface alteration, particle agglomeration/flocculation
그리고 colloidal stability 에 대한 정보를 직접으로 확인할 수 있다. 그리고 dispersion state 를 정량화 할 수 있으며 formulation 을 최적화 할 수 있다.
전통적 방법으로 측정된 zeta potential 은 agglomeration/flocculation 을 정확하게 반영하지 못하고 특히 soft particle 의 경우 zeta potential 값이
충분히 큰데도 불구하고 flocculation 될 수 있다.
|
|







