 |
특허받은 membrane hydrophilization 공정을 이용하여 filter 표면을
친수성으로 만든 필터로서 이 결과 filter 는 다음 두 가지 특징을 가지게 된다.
- wettability 가 매우 탁월
- protein 이 임의로 filter 에 binding 하는 것이 매우 감소 |
|
 |
filter 의 wettability 가 크게 향상됨에 따라 filter 를 flushing 시키는
물의 양을 95% 나 절약할 수 있다. |
|
 |
flushing 하는 물의 양을 많이 절약할 수 있는 특징으로 인해 비용과 시간이 절약된다. |
|
 |
filtration 능력이 크게 향상되어 필터 사용량을 절약 가능. |
|
 |
membrane 의 wettability 가 크게 향상되었으므로 filter 를 신뢰성을 테스트하는 integrity testing 에 대한
신뢰도가 크게 향상됨 |
|
 |
PES membrane 의 surface 를 친수성으로 변환하였으므로 임의로 membrane 표면에 binding 하는
protein 의 양이 대폭 줄어들어 product yield 가 최대로 향상 |
|
 |
double layer 구조의 filter (0.45um + 0.2um)
- pre-filter 기능을 하는 0.45um pore size 인 layer
- 최종 filtration 기능을 하는 0.2um pore size 의 sterilizing-grade filter |
|
 |
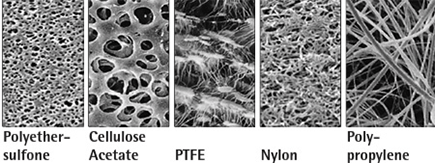
ultrafiltration, microfiltration 등의 목적으로 가장 널리 사용되는 PES (polyethersulfonic) 재질의 filter |
|
| |
 |
|
 |
filter 내부가 asymmetry (비대칭구조) 로 되어 대량의 media 를 filtration 가능한 필터 |
|
| |
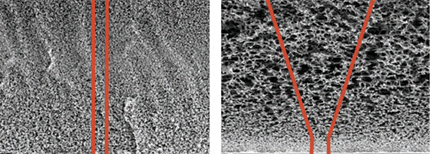
필터의 대칭 및 비대칭 구조 |
|
 |
coarser pre-filter 가 일차적인 filter 를 진행하므로 전체적으로 filtration 용량이 대폭 증가하게 되어 filtration 공정에서 비용이 50 - 90% 절감됨 |
|
 |
Applications
- monoclonal antibody 와 같은 고가의 바이오제품의 생산
- 일회용 filtration 공정에 적합
- blodd & plasma 공정
- Ophthalmics (안과용 액제) 생산 등 |
|
 |
Regulatory Compliance |
|
- Bubble Point Test & Diffusion Test
- ASTM current F-838 guidelines
- ISO 9001 certified Quality Management System
- WFI quality standards bycurrent USP
- Non pyrogenic by USP Bacterial Endotoxin
- USP Plastic Class VI Test
- Non fiber releasing according to 21 CFR |
|
| ※ |
기타 상세한 내용은 영진코퍼레이션 학술기획팀 문의 바랍니다. |
|







