REAL-TIME & REAL-CONDITION CHARACTERIZATION OF ADJUVANTS AND VACCINES
실 시간으로 실제 또는 가속 조건에서 분산 안정성을 측정하는 분석 기기’를 제공하는 독일 LUM사의 웹 세미나가 최근 진행되었습니다. 세미나 내용 중, LUM사가 공급하는 장비 중에 하나인 LUMiReader PSA를 사용한 vaccine adjuvants 관련 case study를 소개합니다.

2011년에 작성된 WHO Technical Report Series 962, WHO Expert Committee on Biological Standardization Fifty-seventh repor (Annex 3)에 ‘Guidelines on stability evaluation of vaccines’이
포함되어 있으며 다음 내용을 설명하고 있습니다:
(1) 백신의 생산과 사용의 각 단계에서 안정성 평가,
(2) 백신의 안정성 평가와 관련된 규제 사항,
(3) 실험 계획과 통계적 고려 사항,
(4) 데이터 분석,
(5) combined vaccines의 안정성 평가,
(6) label 방법.
Accelerated stability studies
위의 내용 중 accelerated (가속화) stability studies에 대한 내용을 살펴보면, 장기적인 안정성을판단하기 위해서 accelerated degradation test의 데이터를 활용할 수 있습니다.
또한 권고 저장 온도보다 높은 온도에 노출된 결과로써 일정 시간 동안 백신의 품질 변화율을 결정하는 연구가 가능합니다. 또한 이 연구는 초기 개발 단계에서 백신의 안정성에 대한
preliminary information을 제공하고 생산 공정 변화 후 백신의 안정성을 평가하는데 활용할 수 있습니다. 그러나 이 연구는 유통기한이나 출시사양을 설정하는데 support data로 사용될 수 있지만,
백신의 real-time & real-condition stability를 예측하는데 사용되어서는 안됩니다.
Real-time & real-condition stability study of formulations with Al-adjuvants
예상 관리/저장 조건에서 예상 유통/저장 기간동안 백신의 물리학적, 화학적, 생물학적, 생물약제학적, 미생물학적 특성에 대한 실시간 & 실조건 안정성에 대한 연구는 저장 조건을 제안하고
유통기한과 출시사양을 설정하기 위해 사용됩니다.
웹 세미나에서는 aluminum adjuvants의 실시간 & 실조건 안정성 연구 사례가 소개되었습니다.


실험 1: Different redispersing of one sample
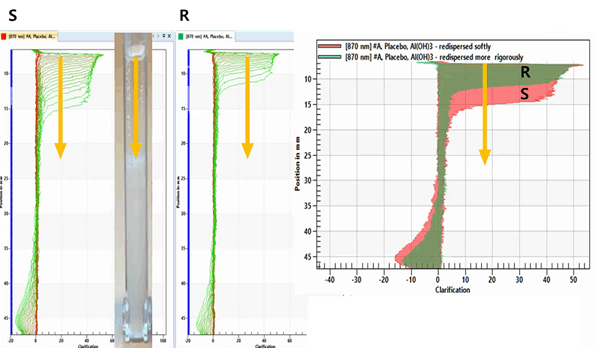
Al(OH)3에 protein을 load하지 않은 Placebo 샘플을 측정 전에 2가지 조건의 강도로 shaking하여 준비하였습니다: 약하게 redispersing (S), 강하게 redispersing (R).
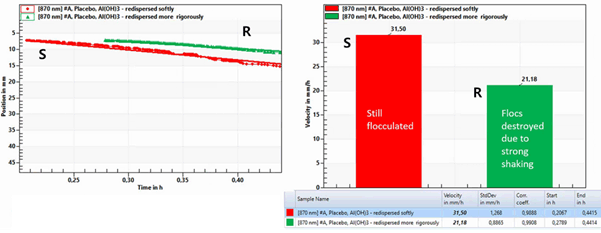
아래 LUMiReader PSA 측정 결과를 보시면 shaking 강도에 따라 다르게 침강한 것을 볼 수 있습니다. 샘플 R은 입자의 침전 속도가 상대적으로 더 느리게 나타났으며,
샘플 S는 상대적으로 침전 속도가 빠르게 나타났습니다. S는 여전히 응집체가 있었으나, R은 강한 shaking으로 인해 응집체가 파괴된 것을 알 수 있었습니다.
이 결과는 독일 LUM사의 분산안정성 측정 기기인 LUMiReader PSA를 사용하였을 때 energy entry가 샘플에 주는 영향과 re-dispersibility의 정도를 측정할 수 있음을 보여주는 것이었습니다.
실험 2: Repeated redispersing of one sample with same intensity
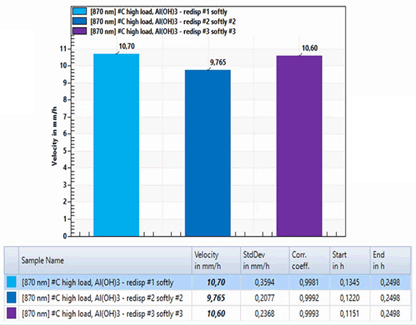
먼저 Al(OH)3에 protein을 high loading한 샘플을 준비하였습니다. 이 샘플을 모두 약한 강도로 동일하게 shaking 해주고 이 샘플들의 분산 안정성을 LUMiReader PSA로 측정하였습니다.
이와 같은 반복 실험으로 이 기기를 통해 재현 가능한 분산 안정성 분석 결과를 얻을 수 있는지 알아보았습니다.
아래 그래프에서 볼 수 있는 바와 같이 LUM사에서 제공하는 전용 소프트웨어로 분석하였을 때, 제공된 분석 방법 중 Front Tracking 분석 결과에 의해 침강 속도를 결정할 수 있었습니다.
3회 반복 실험 결과는 유의적인 차이를 보이지 않아 이 기기의 측정이 재현가능한 결과임을 알 수 있었습니다.
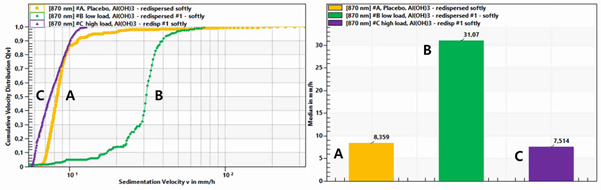
실험 3: Sedimentation velocity of three different samples with same redispersing
Al(OH)3에 protein을 loading하지 않거나 (A), low loading (B), high loading (C) 하여, 이 세 시료를 각각 약하게 shaking하여 redispersed 시켜 준비하였습니다.
아래 왼쪽 그래프를 보시면 각 formulation 내의 침강 입자의 속도가 시료마다 다르게 나타남을 알 수 있었으며, loaded protein이 침강에 미치는 영향을 정량화 할 수 있었습니다.
Summary
Multiwavelength - Separation Anayser - LUMiReader® PSA 452는 vaccine과 adjuvants 연구에서 다음과 같이 사용될 수 있습니다.
 WHO guidance의 권고를 따른 ‘Real-time & real-condision stability’ 연구에서 분산액의 물리적인 특성을 WHO guidance의 권고를 따른 ‘Real-time & real-condision stability’ 연구에서 분산액의 물리적인 특성을
직접적으로 알 수 있으며,
물리적 특성에 영향을 주는 요인에 대한 정보를 간접적으로 알 수 있습니다.
 WHO guidance의 기준에 부합한 조건 (4~80℃)에서 ‘Accelerated stability studies’를 할 수 있습니다 WHO guidance의 기준에 부합한 조건 (4~80℃)에서 ‘Accelerated stability studies’를 할 수 있습니다
 샘플의 원래 농도 수준에서 분산 안정성을 확인할 수 있습니다. 샘플의 원래 농도 수준에서 분산 안정성을 확인할 수 있습니다.
 그 외에도 다음과 같은 더 많은 목적으로 LUMiReader를 사용할 수 있습니다. 그 외에도 다음과 같은 더 많은 목적으로 LUMiReader를 사용할 수 있습니다.
|
|
|

LumiSizer Dispersion Analyzer

LumiFuge Stability Analyzer
LumiReader PSA Separation Analyzer
|
|